Patented Tracer-QC™ Technology
The biggest challenge with automation of radio-pharmaceutical QC is that it requires a set of very different tests which rely on conceptually different pieces of equipment or individual manual procedures. Trace-Ability has solved this challenge by focusing on the answers the tests need to provide. Rather than developing new tools to carry out known tests, we have developed new tests that can be run on a single known tool and yield the needed answers. The tool is a plate reader, all tests are based on assays that yield optical signals measurable by the plate reader.
Product
Tracer-QC system consists of a plate reader with a pipetting robot on top. It is enabled by innovative consumable kits. The user installs the kit into the instrument and delivers a single PET tracer sample in a shielded vial. The pipettor then mixes this sample and standards with indicators and moves the resulting mixtures to the analysis plate inside the plate reader to measure optical signals that are generated by interactions of the sample with either indicators or features of the analysis plate. The signals are interpreted as measurements of all required QC parameters and complied into a comprehensive report by 21 CFR Part 11-compliant software. Once the process is over, the kit can be removed and the system is ready for the next procedure because the sample or reagents never come in contact with the plate reader.
Tracer-QC rHPLC configuration include a completely integrated HPLC, which is operated in an automated manner. Tracer-QC robot performs injections of sample and standards and Tracer-QC software analyzes the chromatograms to yield the desired QC measurements.
Tracer-QC allows complete hands-free operation from a single sample to a full QC report.
Tracer-QC instrument is shielded while the sample is delivered via a keyed pig. Thus, the operator never handles or has a direct line of sight with an unshielded sample.


Complete Turn-Key Solution
Offered in partnership with LabLogic:
- Instruments
- Kits
- Training
- Service
- Support

The process
1. Install the kit
User scans the QR code on the kit to load the correct analysis program and installs the consumables from Tracer-QC kit onto the Tracer-QC instrument.

Tracer-QC confirms that the kit was installed properly, executes automated self-checks and indicates that it is ready to receive the sample.
Tracer-QC robot mixes the sample with indicators and dispenses the mixtures into the analysis plate. The plate reader analyses the optical signals (absorbed or emitted light) in the analysis plate.
2. Deliver the sample

The sample vial provided in the kit is placed inside a special pig that provides shielding and is designed to mate to Tracer-QC. QC sample is added to the vial in the pig from a QC syringe inside a hot cell. The pig is then carried to Tracer-QC and placed on the deck behind shielding where it is accessible by the pipetting robot.
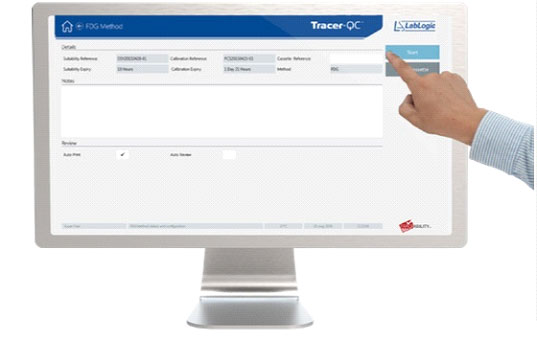
3. Initiate the analysis
User initiates the analysis using the software
Tracer-QC robot mixes the sample with indicators and dispenses the mixtures into the analysis plate. The plate reader analyses the optical signals (absorbed or emitted light) in the analysis plate.

The software then analyses their optical characteristics against a set of predefined and validated reference specifications and translates them into the required QC measurements.
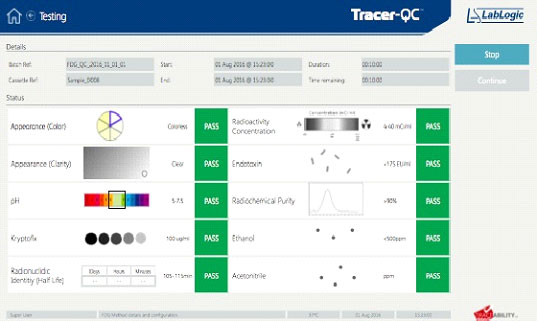
4. Collect the report
A single page summary is delivered automatically with all QC values and pass/fail determination supported by a detailed 26-page report with all measurements of sample and standard obtained during the process as well as suitability test data.

Features & Benefits
Ease of Compliance
No room for missing a process, record or signature
- Objective measurements with reference to standards and without any human interaction
- Data flow from measurement to batch record
— Automated
— Uncompromised
— Completely traceable
- Regular automated suitability testing with permanent record
- 21 CFR 11 compliant software with access control
- No cross-contamination samples never leave disposable kit (except rHPLC))
- Ease of audit (internal or FDA) – instantaneous data retrieval
Efficiency and Safety
- 1 electronic report with all QC results, automatically generated
- Rapid QC results
- Increased throughput (to enable scale-up)
- Reduced risk of radioactive spills and contamination
- Reduced personnel exposure
- No cleaning or equilibration required (disposable kit)
- Inventory reduction (tracking individual expiry dates of multiple supplies/standards)
- Process standardization across sites and/or products
Cost Reduction
- Fewer and less skilled personnel
- Faster, cheaper training
- Faster, cheaper audits
- Remote record access/auditing
- Avoid cost of addressing 483’s
- One machine to maintain
- Estimated net savings ~$26,000/year for FDG production (most common tracer)
- Estimated net savings of $56,000/year for each additional tracer beyond FDG
Size
- 34 cm (W), 98 cm (H), 67 cm (D)
- Takes up as much room as a typical GC
Scalability
- Platform for easy addition of other tracers
One instrument
- Single mode of detection for all tests
Contactless Testing
- Sample never touches the instrument
Disposable Kits
- Single use, completely disposable path
